Power Supplies for Medical Applications – everything you need to know about IEC 60601 and 2 x MOPP
Follow articleHow do you feel about this article? Help us to provide better content for you.
Thank you! Your feedback has been received.
There was a problem submitting your feedback, please try again later.
What do you think of this article?
People and power don’t really go together, especially when people are medical patients. Healthcare devices are heavily regulated by standards-based requirements and associated product testing, to protect patients and healthcare professionals.
The most important standard is IEC 60601, which consists of a range of requirements for the electrical and electronic equipment used in healthcare. First published around 40 years ago, IEC 60601 has kept up with industry changes.
This article explains the main principles of IEC 60601 with respect to the supply of power and focusses on new requirements, such as the need for risk assessment. Additionally, it will review practical steps for achieving compliance and the support that is available for medical device manufacturers.
MOOPs and MOPPs
One of the key safety concerns with respect to medical devices is that the patient is often electrically connected to the device. Consider, for example, the conductive pads of an electrocardiograph. In IEC 60601 the connected parts are defined as ‘applied parts’ (AP) - an important definition within the standard when specifying a medical product’s overall requirements.
According to IEC 60601, it is essential that medical devices incorporate at least one Means of Protection (MOP) to ensure that both the patient, if connected via an AP, and operator are protected from the risk of electric shock, even under fault conditions. This can be achieved through safety insulation, protective earth, a defined creepage distance, an air gap, other protective impedances, or by putting in place a combination of these techniques.
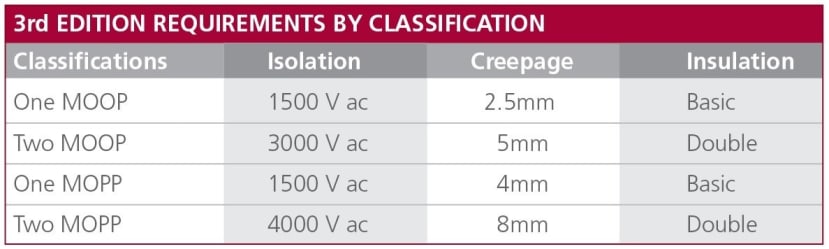
The standard treats operators and patients slightly differently, resulting in different classifications: ‘Means of Operator Protection’ (MOOP) and ‘Means of Patient Protection’ (MOPP). One reason for this differentiation is that the patient may be physically connected via an AP and unconscious when the electrical fault happens. This means that the MOPP requirements are more stringent than MOOP. Each term is defined in terms of isolation voltage, creepage distance, and insulation level.
Figure 1: Definitions of MOOPs and MOPPs for IEC 60601
IEC 60601 – Always Evolving
Since its first publication 40 years ago IEC 60601 has evolved. Power supplies are not medical devices in their own right so the standards do not apply to them directly. What is critical, however, is that medical device manufacturers would not be able to achieve compliance without power solutions that have been designed specifically to carry out medical applications.
Until recently, it was a safe assumption that medical devices would be used exclusively in medical facilities, such as hospitals and clinics, which provided dedicated clean power for use by their most sensitive medical devices. The situation has changed and nowadays it is common for patients to use medical devices at home. This means that issues such as electromagnetic compatibility (EMC) take on greater importance, in part due to the growth of technologies such as Bluetooth and Wi-Fi. As a result, the most recent version of the standard (4th edition) has changed testing procedures and acceptance levels for EMC.
Another significant change has been the introduction of the requirement to carry out a risk assessment in accordance with ISO 14971. Risk management is a key component in demonstrating regulatory compliance for medical devices - ISO 14971 defines best practices for all managing a medical device through every stage of life.
The Medical Device Directive also requires manufacturers to implement a Quality Management System (QMS) compliant with ISO 13485. The primary requirement for a power supply manufacturer is that they are able to demonstrate their ability to meet both customer and regulatory requirements on a consistent basis.
2 x MOPP rated DC/DC converters … improved flexibility to achieve MOPP protection
Usually, when working to achieve IEC 60601 compliance, manufacturers use an AC/DC power supply approved for medical use. However, BF rated applications also require the AP instrument to meet the 2 x MOPP rating – something which many of the medically approved AC/DC power supplies on the market today do not have, meaning they don’t offer appropriate stand-alone power solutions for applications where BF compliance is required. However, an IEC 60601 approved, 2 x MOPP rated DC/DC converter will support BF compliance for the AP. Another common example is medical appliances that implement a battery back-up capability and must fulfil the 2 x MOPP rating during AC failure.
Medical appliances often require various DC voltages driving the AP instrument that differ from the main system DC voltage provided. Manufacturers can avoid having to source a custom AC/DC power supply by combining IEC 60601, 2 x MOPP rated DC/DC converters together with, for example, an ITE 60950 rated AC/DC power supply. In other instances, an alternative is to use 2 x MOPP, medical grade DC/DC converters for the AP instrument, even when the selected AC/DC power supply has IEC 60601 approval.
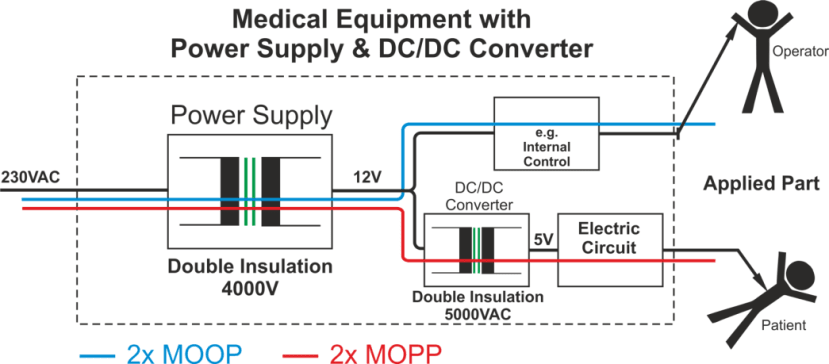
Figure 2 below indicates the key requirements for achieving 2 x MOPP – these are 4000 V AC isolation, 8mm creepage distance and double insulation. Most DC/DC converters which are commonly available (including those with EN60950 approval) offer between 500 V DC and around 1600 V DC of isolation, meaning that they are not suitable for medical applications. However, specialised DC/DC converters are available that will meet the requirements for AP when used in conjunction with such standard off-the-shelf power supplies.
By providing up to 5000 V AC of isolation, double insulation and 8mm of creepage distance through its galvanically-isolating transformer, a DC/DC converter can still protect the patient in the event of a failure of the main AC/DC power supply, thereby avoiding mains voltage levels appearing at any patient AP points.
Figure 2: Using a DC/DC converter to achieve 2 x MOPP protection
TRACO POWER – a leader in medically safe power solutions
Transformer technology sits at the heart of Traco’s approach to delivering world-class power solutions for the medical industry. The company have developed and honed a unique approach which ensures required levels of separation and isolation, while still achieving sufficient coupling to allow the DC/DC converter to operate efficiently.
Low coupling capacitance between primary and secondary transformer windings is an important aspect of the protection implementation. Values as low as 10-15pF ensure that there is a negligible transfer of current across the isolation barrier, providing patient protection in line with the requirements of the IEC 60601.
Traco also implement quality management in accordance with ISO 13485, which covers their design and manufacturing processes. As a manufacturer of power solutions, rather than medical devices, Traco is not required to provide risk assessment data. However, the company is compliant with ISO 14971 and provides risk assessment files covering areas such as insulation breakdown, use while inverted, effects of fan failure, flammability, mechanical shock and more to their customers. Providing this data assists Traco’s customers with their risk assessment of final medical products, saving them both time and expense during their design process.
A full selection of 2 x MOPP approved solutions
Traco offers a comprehensive selection of both AC/DC and DC/DC solutions for medical applications, all of which fulfil the 2 x MOPP requirement. Compliant with the EMC requirements of IEC 60601-1 (4th edition), they are suitable for all patient connected, applied parts medical devices (BF compliant). The AC/DC products range from small 5W PCB-mounting modules, a range of mid-power open frame designs, and enclosed power supplies offering power levels of up to 450W.
Traco’s TPP 40 series is available as both open frame and enclosed
All of the PSUs offer universal mains input (85-264VAC / 120-370VDC) with active power factor correction (PFC) above 100W. The range comprises of single, dual and triple outputs, covering almost all application requirements.
The TRACO POWER DC/DC converter range comprises PCB-mounting modules with power levels from 2W to 30W. Devices offer both 2:1 and 4:1 input ranges with 5V, 12V, 24V and 48V nominal inputs available. Single and dual outputs are offered from 3.3VDC to ±15VDC.
The 15 Watt THM series is a PCB-mount medical DC/DC converter