Medtronic shares PB560 Ventilator specifications, design files, BOM and CAD
Follow articleHow do you feel about this article? Help us to provide better content for you.
Thank you! Your feedback has been received.
There was a problem submitting your feedback, please try again later.
What do you think of this article?
Medical device giant Medtronic has recently released specifications and design files for its commercially available PB560 Portable Ventilator in an effort to inspire and support those who are working on their own ventilator designs in the fight against COVID-19. Sharing such detailed information of a commercial product is quite groundbreaking - particularly amongst medical device manufacturers where protection of IP is a high priority. Whether you are working on developing your own ventilator design, such as the OxVent or MIT Emergency Ventilator (E-Vent) project or not, these files provide an interesting insight into the level of documentation required to manufacture and sell a medical-grade device.
What has been shared?
Since announcing their ambition to share the design Medtronic has released three archives, providing increasingly detailed information. Here is a summary:
Medtronic PB560 Ventilator System – Release 1.0
- Electrical schematics
- Manuals
- Manufacturing documents
- Requirements documents
Medtronic PB560 Ventilator System – Release 2.0
- Manufacturing fixtures
- Printed circuit board drawings (including multiple BOMs)
- 3D CAD files
- Mechanical part drawings
Medtronic PB560 Ventilator System – Release 3.0
- Source code files
Taking a closer look
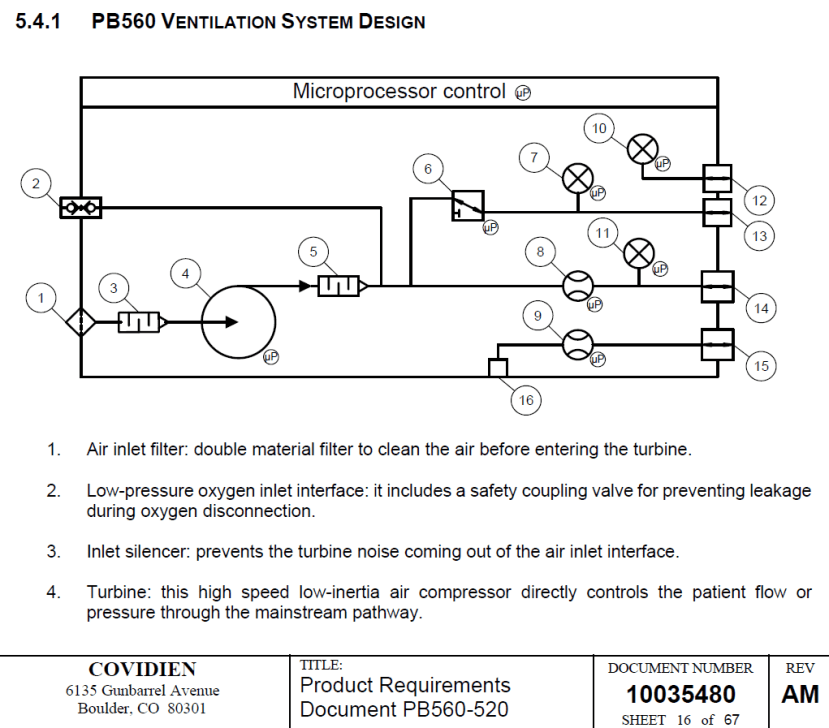
Requirements
The requirement documents set out detailed specifications for the system. These include performance, control, safety and physical requirements for the device. Below is an excerpt from the Product Requirements Document detailing a block diagram for the ventilation system.
Example of Ventilator Product Requirements
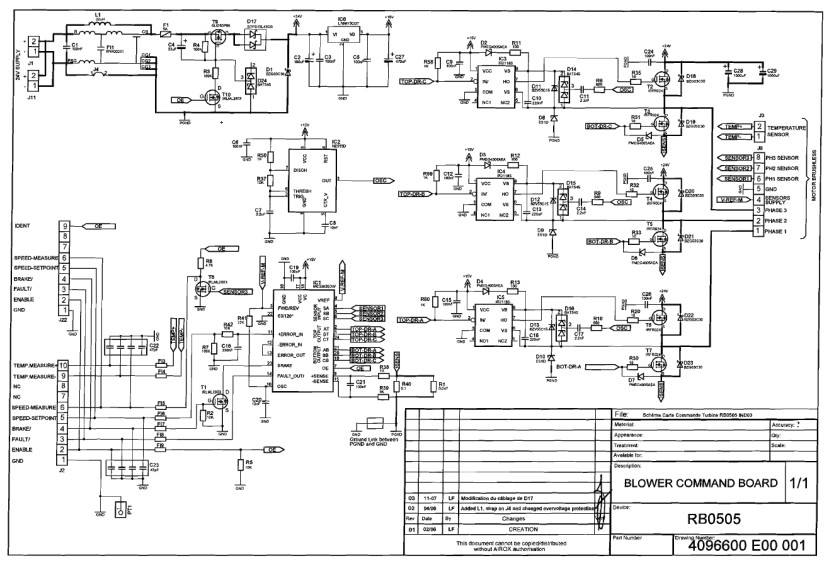
Design
Mechanical CAD, electronics schematics and PCB layouts are also included in the release which provides useful insight into how the ventilator control system works with a combination of electronics and mechanical fail safes.
Blower controller PCB schematic
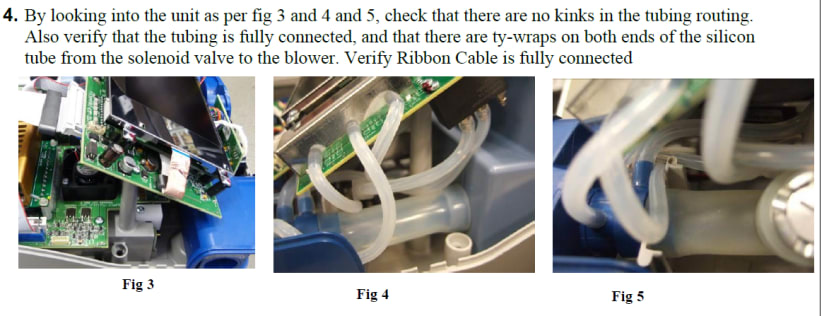
Assembly
Detailed assembly instructions are included which make up part of the products Quality Management Process. A robust Quality Management System is essential for medical devices manufacturers who must be able to prove that measures are in place to ensure high-quality products can be repeatedly produced at scale. Documented tests and procedures must be followed to ensure all products are safe for use with patients and will continue to work reliably over the products expected lifespan.
Example of the product assembly procedure
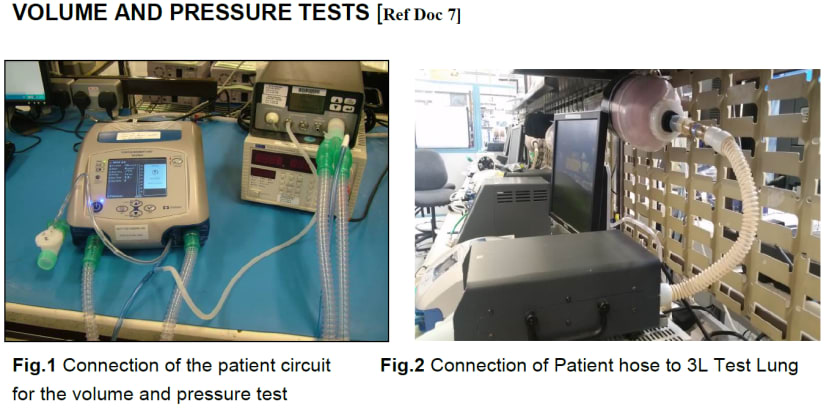
Testing
Detailed test procedures are described for the product which is used to validate its performance prior to shipping. These testing documents describe the test setup and acceptable bounds for results.
Example of test procedures
Components
The PB560 is a relatively old design, having been first released in 2010 therefore some of the components listed in the Bill-of-Materials are now difficult to source. The main processor is an ST Microelectronics ST10F276Z5T3, other key components include the Honeywell AWM3300V airflow sensor and Texas Instruments BQ2002CSN battery charge controller.
Firmware
In the latest release, Medtronic has provided full source code files for the device. These might be particularly interesting if you want to know what is required to build software for certified and robust medical devices.
Excerpt of VEN_Compute.c
Let us know what you think!
You can sign up to access the design files from Medtronic here. Take a look and let us know what you think of this bold step by Medtronic in the comments!



Comments