A Microfluidic System for In-Vitro Culture of Liver Tissue
Follow projectHow do you feel about this article? Help us to provide better content for you.
Thank you! Your feedback has been received.
There was a problem submitting your feedback, please try again later.
What do you think of this article?
 This project focuses on designing a microfluidic system for in-vitro culturing of liver tissue slices from the biopsy samples of cancer patients. The platform enables drug testing within a clinical setting, accelerating drug development timelines.
This project focuses on designing a microfluidic system for in-vitro culturing of liver tissue slices from the biopsy samples of cancer patients. The platform enables drug testing within a clinical setting, accelerating drug development timelines.
Background and Problem
Cancer research is often associated with expensive lab equipment, highly skilled personnel and specialized facilities. Precision medicine technologies such as microfluidics promise to bridge the gap between the hospital and the laboratory by reducing sample volumes and allowing non-technical clinicians to perform laboratory tests at the patient’s bedside.
Integrating each patient’s unique medical profile (including genetics and underlying conditions) into the treatment plan allows doctors to deliver optimum personalized care to individual patients. This is especially important in cancer treatment due to the large amount of variation between different tumours and even individual cells within a single tumour.
Surgical extraction of biopsy material directly from the disease site can allow scientists to identify the optimum combination and dosages of drugs required to achieve the best patient outcomes for different tumour types. However, this often requires keeping microtissue samples alive and healthy outside the patient’s body for prolonged drug testing. Some of the greatest challenges of this task include: 1) providing physiologically relevant conditions to recreate the ideal cancer microenvironment outside the body, and 2) maintaining the cellular architecture of the sample’s native state by minimising cellular reorganization.
Aims and Objectives
The main goal of this 6-month project is to design a microfluidic platform capable of sustaining precision-cut liver tissue slices in an in-vitro culture for up to 5 days while preserving tissue viability and function.
This article will showcase the design of a custom incubation circuit and a multiplex oxygenator to complement the microfluidic platform. The purpose of the circuit is to maintain the temperature of the microfluidic system at 37°C throughout the culture duration. The oxygenator is required to saturate the perfusion culture medium with a special gas mixture (Carbogen - 95% O2 : 5% CO2) to maximise oxygen delivery while maintaining a stable medium pH level.
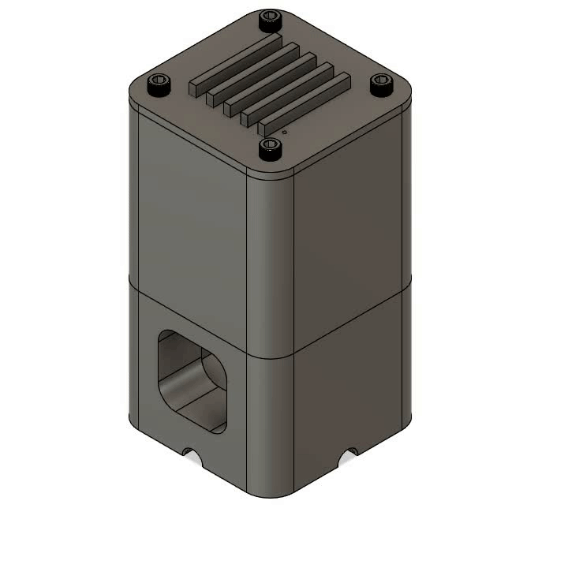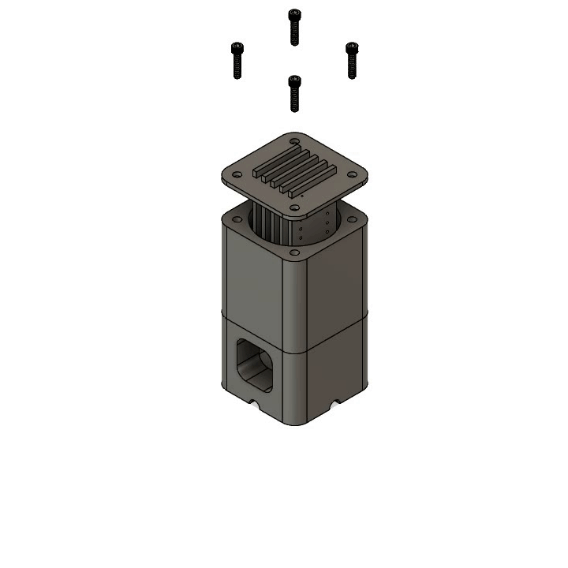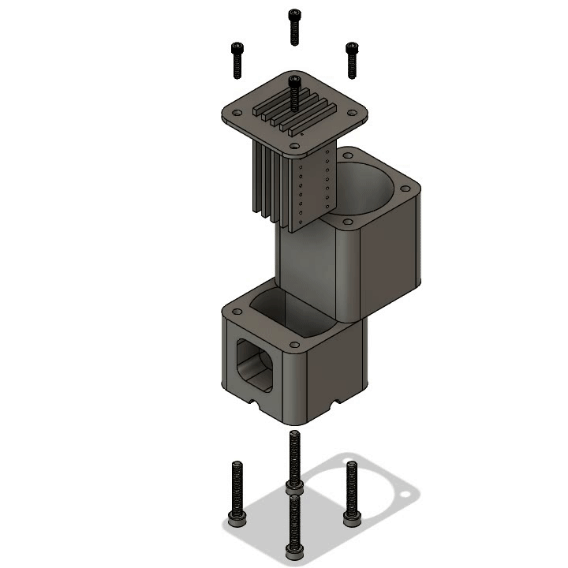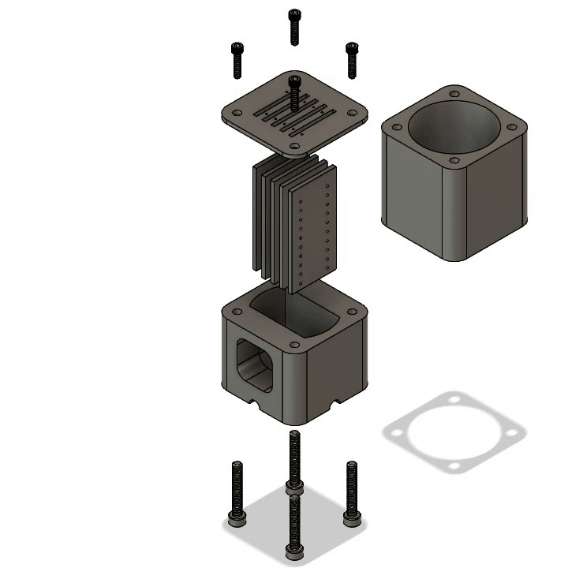
Multiplex Oxygenator
Microfluidic oxygenators operate on the basic principle of diffusion, where oxygen in the gaseous phase dissolves in the circulating medium upon travelling through a gas-permeable membrane. Polytetrafluoroethylene (PTFE) tubing was used as the diffusion membrane due to its simplicity, sufficient gas permeability and low cost. The diameter was chosen to match the rest of the fluidic system to allow robust fluidic connections.

The main novelty of this design over traditional oxygenators is its multiplex efficiency. The modular 3D printed system allows up to 5 oxygenation cartridges to be inserted, enabling 5 independent fluidic lines (each 25cm long) to be oxygenated within a 46ml gas volume. The high surface area to volume design provides multiple benefits including 1) reduced consumption of Carbogen, 2) less workspace required for operation and 3) low cost and rapid replacement of cartridges for sterile experiments via laser cutting. A deionised water reservoir is used on the base of the oxygenator to provide pressure resistance to the Carbogen supply, further reducing the consumption of the precious gas mixture.
The prototype oxygenator was used in primary culture for 48 hours for initial testing. Further characterisation of oxygenation is planned in due course using a fibre-optic oxygen sensor.
Circuit Schematic
A closed-loop control circuit based around the commercial Arduino Micro was designed for prototyping and testing purposes using the Altium Designer software.
The circuit maximizes the operational capacity of the Arduino Micro by using all 6 of its pulse width modulation (PWM) pins for driving Peltier heating elements. This will allow up to 4 microfluidic chips as well as the multiplexed oxygenator to be maintained at 37°C, each controlled by its own temperature feedback loop.
Overshoot of the temperature would be catastrophic in this application due to the highly sensitive nature of the cells as well as the proteins within the culture medium will quickly become denatured. Equally undershoot over prolonged periods would diminish tissue viability due to cell hypothermia. The DS18B20 digital temperature sensor will be used in conjunction with the proportional–integral–derivative (PID) control library to regulate the Peltier output. The sensor will be sterilised and embedded within the cell culture chip to provide accurate temperature readings. An initial biocompatibility test (consisting of a live/dead assay and LDH assay) was carried out to ensure that liver cells are not affected by the presence of the sensors in contact with the culture medium, however further testing is planned for the duration of the project.
The large current required by the Peltier elements can not be directly supplied by the Arduino, therefore the classic TIP120 Darlington transistor was chosen to drive the current. In order to power the Peltiers and the Arduino simultaneously using the same power supply, an on-board DC-DC converter is used to provide the high power, low voltage output for the heating elements.
An LCD display was incorporated to allow real-time readout of the temperature during culture experiments within a wet lab setting while the SD card module will enable long-term data logging over multi-day experiments.
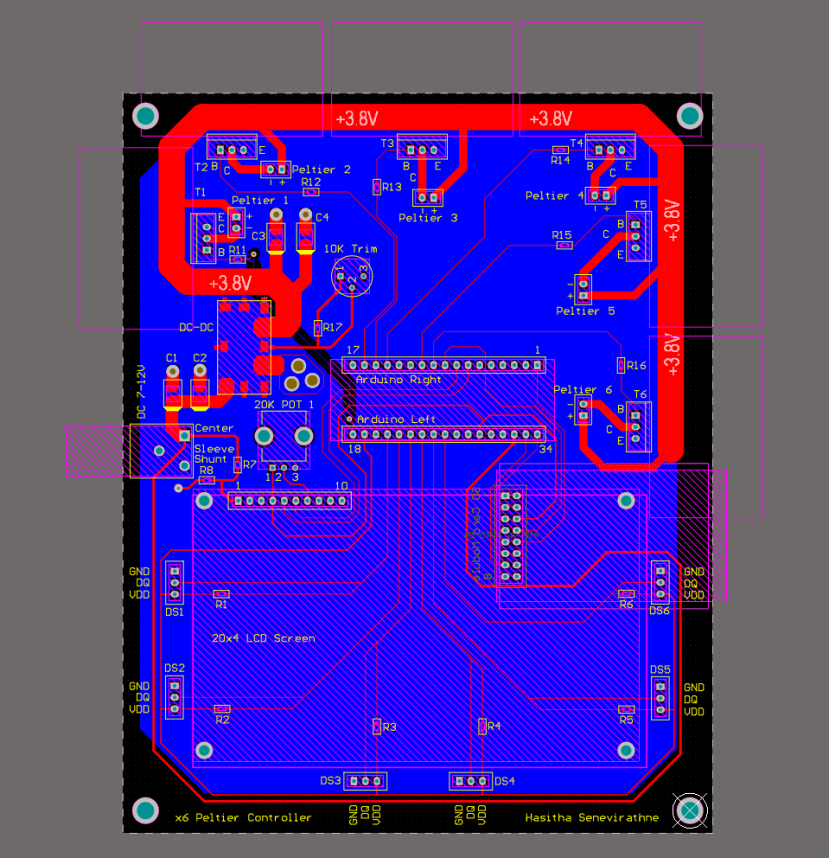
PCB Design
The highly condensed footprint of components and the elimination of free-hanging wires provided ample motivation for designing a PCB of the circuit for use in cell culture experiments. To minimise cost, the circuit was routed on a 2-layer PCB while conforming to the standard conventions of PCB routing.
Next Steps
With the oxygenator complete and the PCB design sent for manufacturing, the focus of the project will shift to characterisation of the complete system assembly. This will be carried out in stages; beginning with electrical/thermal characterisation, followed by biological characterisation with the HepG2 liver cancer cell line and finally primarily liver slices sourced from a collaboration with the University Hospital of Basel. Furthermore, new imaging and staining protocols will be developed for analysis of the viability and morphology of the tissue slices cultured within this new system.